CURRENT PROJECTS:
Molecular-Genetic Origins of Multicellularity
Evolution of the SARS-CoV-2 Virus
Miscellaneous older projects archived here:
Protein Misfolding and Aggregation-Related Disease
In Silico Mechanical Manipulation of Misfolding-Prone Proteins
Insights into DNA Structure and Dynamics Through Coarse-Grained Models
Dielectric Properties of Proteins
Role of Osmolytes and Denaturants on Protein Stability
Dielectric Properties of Proteins
We have developed a theory for the anisotropic and inhomogeneous dielectric properties of proteins. Using a generalization of dielectric theories of polar solids and liquids, we have calculated the mesoscopic, spatially-varying dielectric constant at points in and around a protein by combining the Kirkwood–Fro ̈hlich theory with short all-atom molecular dynamics simulations of equilibrium protein fluctuations. The resulting dielectric permittivity tensor is found to exhibit significant heterogeneity and anisotropy in the protein interior.
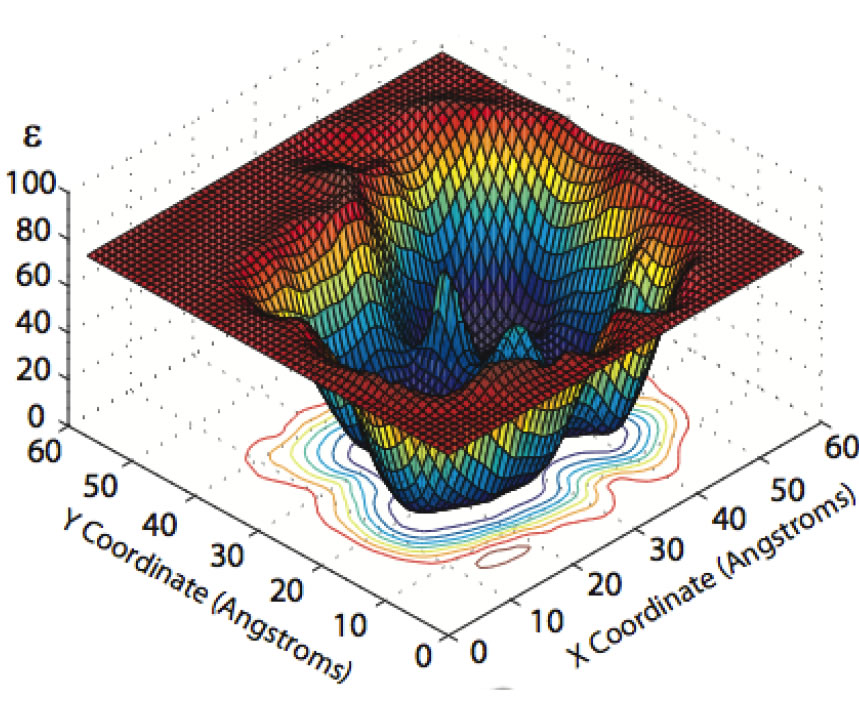
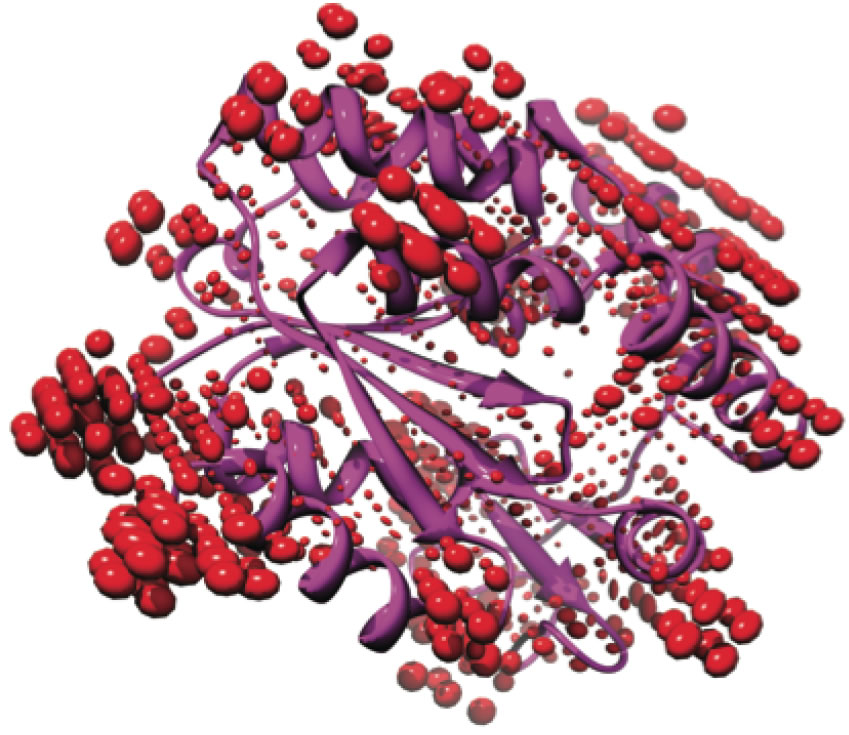
Around the surface of the protein it may exceed the dielectric constant of bulk water, especially near the mobile side chains of polar residues, such as K, N, Q, and E.
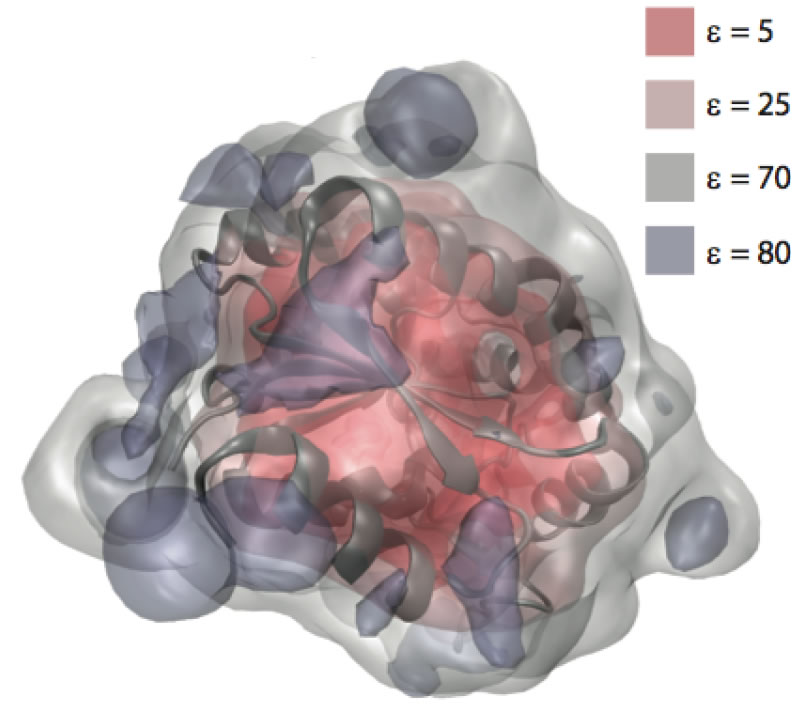
The anisotropic character of the protein dielectric selectively modulates the attractions and repulsions between charged groups in close proximity.
References
Guest W, Cashman NR, Plotkin SS, “A Theory for the Anisotropic and Inhomogeneous Dielectric Properties of Proteins” Phys. Chem. Chem. Phys., 13 (13), 6286 – 6295 (2011)
Guest W, Cashman NR, Plotkin SS, “Electrostatics in the Stability and Misfolding of the Prion Protein: Salt Bridges, Self-Energy, and Solvation” Biochem. Cell Biol 88, 371–381 (2010)